Covid-19 keeping the fun police at bay
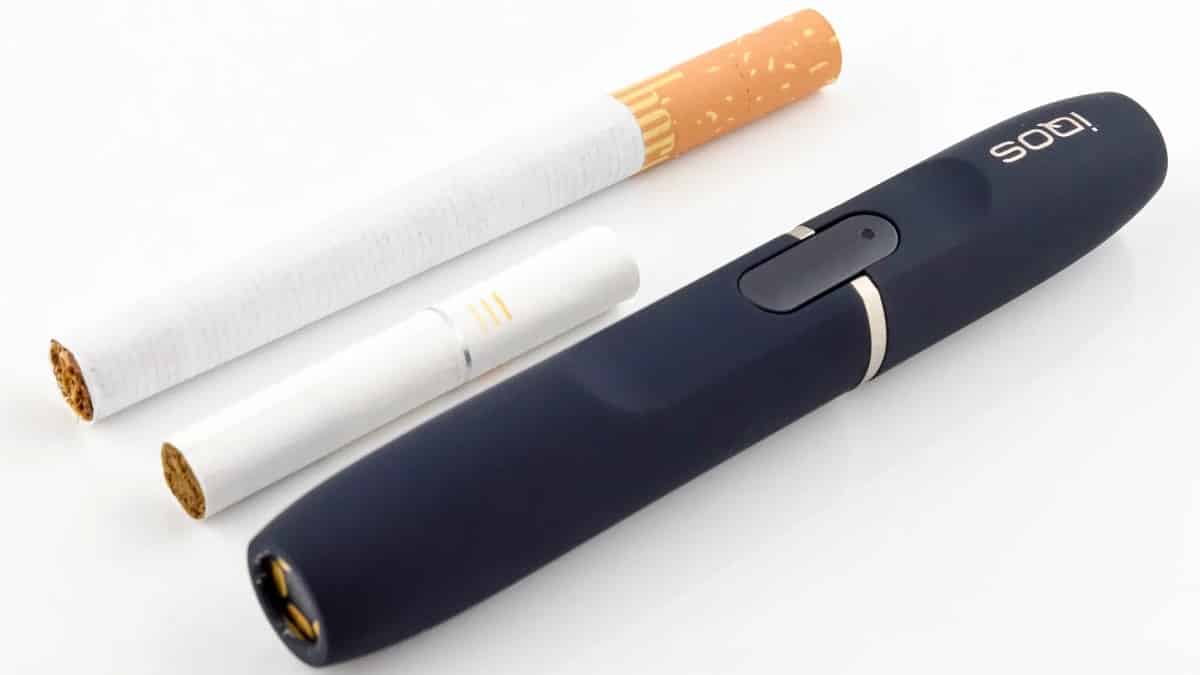
You’ve probably noticed a lack of Heat not Burn news updates recently. There’s a simple reason for that – there’s been a lack of news. The coronavirus pandemic has actually managed to distract a lot of public health experts from trying to ban anything that’s fun, so we don’t have quite as many dubious reports and pointless health campaigns going on. We’ve managed to find a few stories, though, so here they are.








