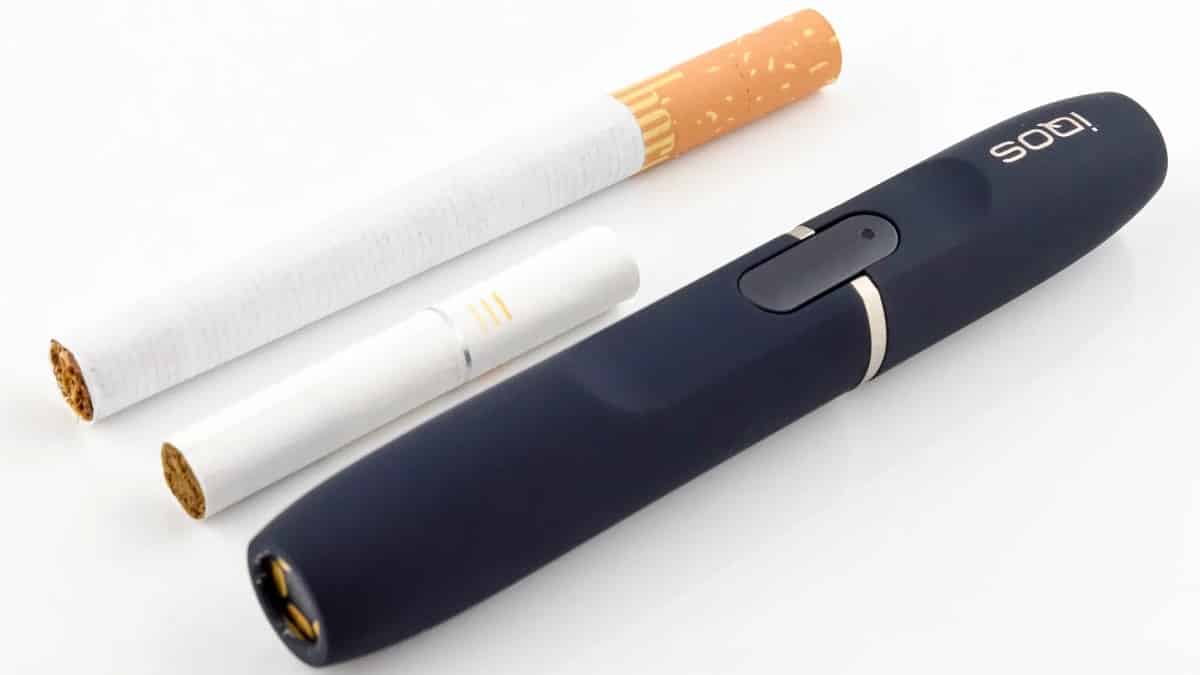
The BAT Glo Hyper Plus is the latest heated tobacco device from British American Tobacco (BAT). As of writing, it is not available in the UK. However, it has been launched amidst much fanfare here in Spain, where it retails at just under ten euros (about eight pounds and fifty pence). The tobacco sticks (more about which later) retail at a price of four euros for twenty.